
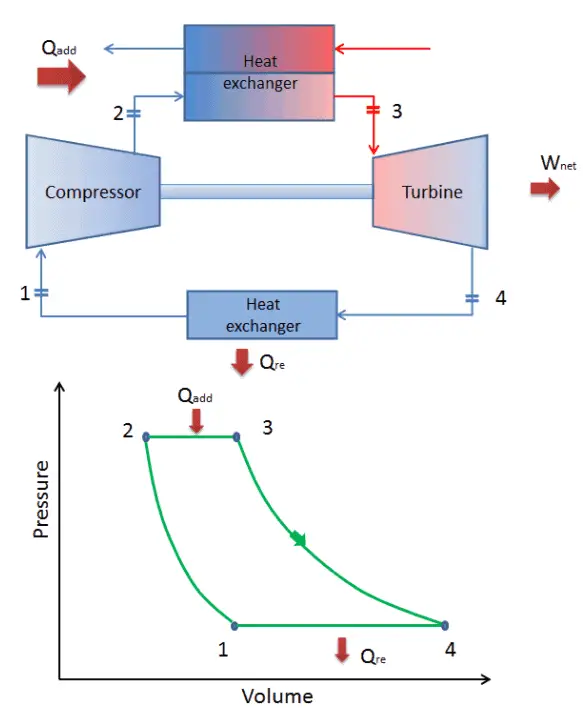
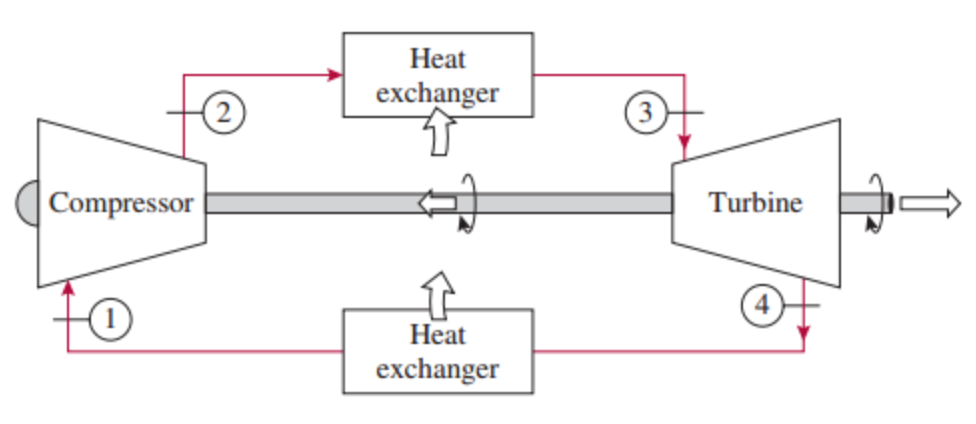
The work done by the turbine is given by W T = H 4 – H 3. The gas works on the surroundings (blades of the turbine) and loses an amount of internal energy equal to the work that leaves the system.
The surroundings work on the gas, increasing its internal energy (temperature) and compressing it (increasing its pressure). Isentropic compression (compression in a compressor) – The working gas (e.g., helium) is compressed adiabatically from state 1 to state 2 by the compressor (usually an axial-flow compressor).
#NET WORKDONE IN BRAYTON CYCLE SERIES#
In a closed ideal Brayton cycle, the system executing the cycle undergoes a series of four processes: two isentropic (reversible adiabatic) processes alternated with two isobaric processes: Modern Combined Cycle Gas Turbine (CCGT) plants, in which the thermodynamic cycle of consists of two power plant cycles (e.g., the Brayton cycle and the Rankine cycle), can achieve a thermal efficiency of around 55%. Since Carnot’s principle states that no engine can be more efficient than a reversible engine ( a Carnot heat engine) operating between the same high temperature and low-temperature reservoirs, a gas turbine based on the Brayton cycle must have lower efficiency than the Carnot efficiency.Ī large single-cycle gas turbine typically produces for example 300 megawatts of electric power and has 35–40% thermal efficiency. In an ideal Brayton cycle, the system executing the cycle undergoes a series of four processes: two isentropic (reversible adiabatic) processes alternated with two isobaric processes. In contrast to the Carnot cycle, the Brayton cycle does not execute isothermal processes because these must be performed very slowly. It is one of the most common thermodynamic cycles found in gas turbine power plants or airplanes. In general, the Brayton cycle describes the workings of a constant-pressure heat engine.
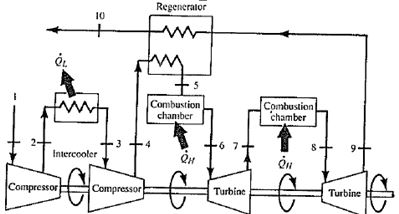
Therefore we describe their thermodynamics by the Brayton cycle. Today, modern gas turbine engines and airbreathing jet engines are also constant-pressure heat engines.
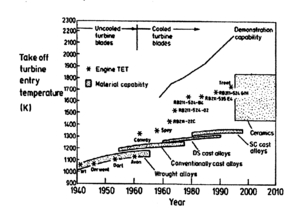
The original Brayton engine used a piston compressor and piston expander instead of a gas turbine and gas compressor. This heat engine is known as “Brayton’s Ready Motor”. In 1872, an American engineer, George Bailey Brayton, advanced the study of heat engines by patenting a constant pressure internal combustion engine, initially using vaporized gas but later using liquid fuels such as kerosene.


 0 kommentar(er)
0 kommentar(er)
